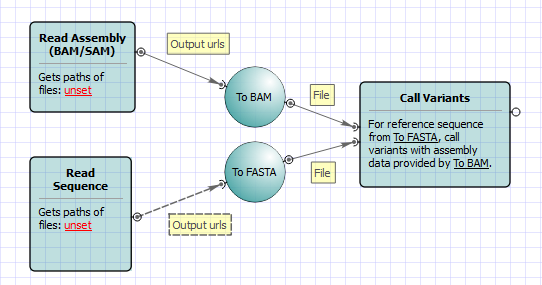
Call variants in UGENE can be done using SAMtools mpileup and bcftools view utilities. To read additional information about SAMtools and its utilities visit SAMtools homepage. Both utilities are embedded into UGENE and there is no need in additional configuration.
To run the worflow you need to select an input reference sequence, a BAM or SAM file and an output file with variations. Optionally, you can change other parameters, for example, set additional parameters of the SAMtools mpileup and bcftools view utilities. Use the workflow wizard to guide you through the parameters setup process. Click Show wizard button on the Workflow Designer toolbar to open it:
The first wizard page appears:
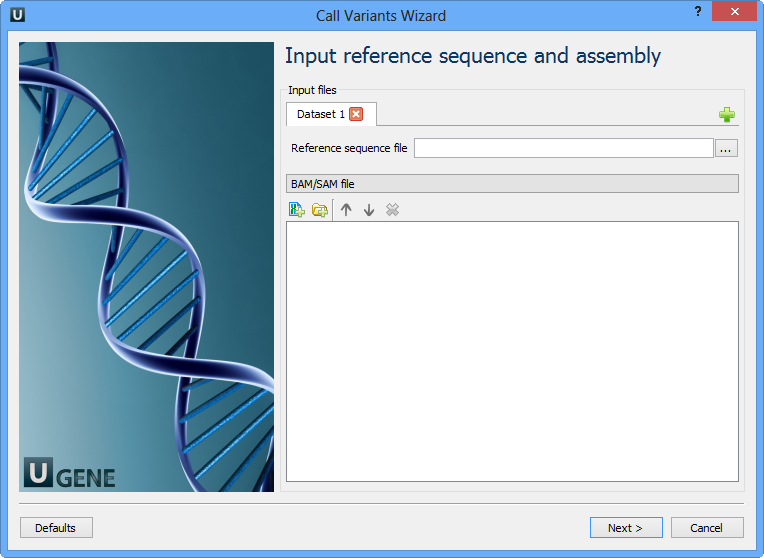
Here you need to input a file with a reference sequence and a sorted BAM or SAM file. Note that the input BAM or SAM file may be unsorted. Click the Next button. The next page appears:
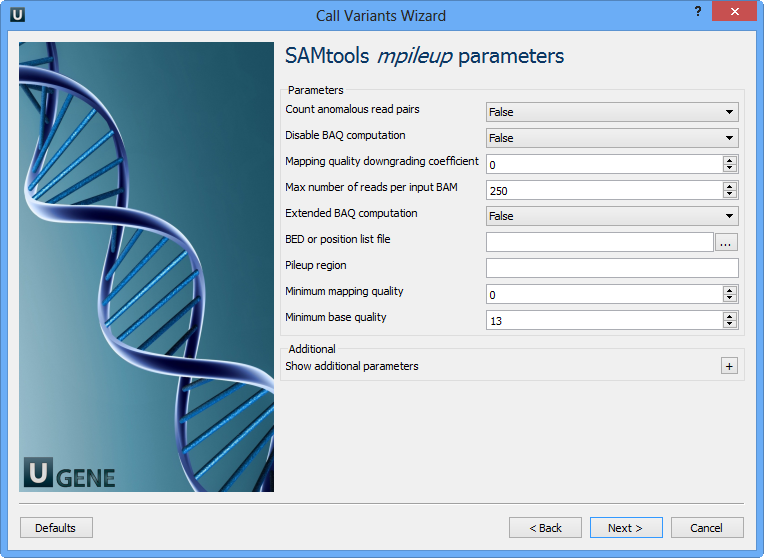
Here you can change default parameters of the SAMtools mpileup utility. To show additional parameters click the + button. The following parameters are available:
Count anomalous read pairs | Do not skip anomalous read pairs in variant calling. |
Disable BAQ computation | Disable probabilistic realignment for the computation of base alignment quality (BAQ). BAQ is the Phred-scaled probability of a read base being misaligned. Applying this option greatly helps to reduce false SNPs caused by misalignments. |
Mapping quality downgroading coefficient | Coefficient for downgrading mapping quality for reads containing excessive mismatches. Given a read with a phred-scaled probability q of being generated from the mapped position, the new mapping quality is about sqrt((INT-q)/INT)*INT. A zero value disables this functionality; if enabled, the recommended value for BWA is 50. |
Max number of reads per input BAM | At a position, read maximally INT reads per input BAM. |
Extended BAQ computation | Extended BAQ computation. This option helps sensitivity especially for MNPs, but may hurt specificity a little bit. |
BED or position list file | BED or position list file containing a list of regions or sites where pileup or BCF should be generated. |
Pileup region | Only generate pileup in region STR. |
Minimum mapping quality | Minimum mapping quality for an alignment to be used. |
Minimum base quality | Minimum base quality for a base to be considered. |
Illumina-1.3+encoding | Assume the quality is in the Illumina 1.3+ encoding. |
Gap extension error | Phred-scaled gap extension sequencing error probability. Reducing INT leads to longer indels. |
Homopolymer errors coefficient | Coefficient for modeling homopolymer errors. Given an l-long homopolymer run, the sequencing error of an indel of size s is modeled as INT*s/l. |
No INDELs | Do not perform INDEL calling. |
Max INDEL depth | Skip INDEL calling if the average per-sample depth is above INT. |
Gap open error | Phred-scaled gap open sequencing error probability. Reducing INT leads to more indel calls. |
List of platforms for indels | Comma dilimited list of platforms (determined by @RG-PL) from which indel candidates are obtained. It is recommended to collect indel candidates from sequencing technologies that have low indel error rate such as ILLUMINA. |
Choose these parameters and click the Next button. The next page appears:
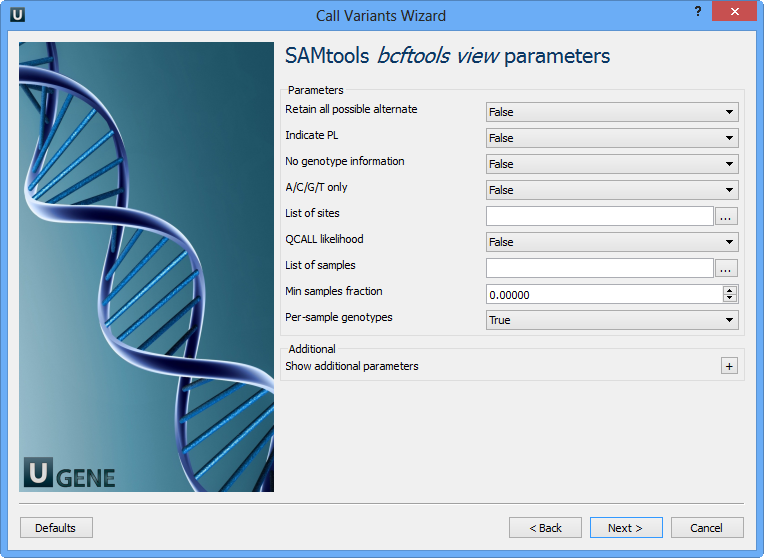
The next page allows one to configure SAMtools bcftools view utility parameters:
Retain all possible alternative | Retain all possible alternate alleles at variant sites. By default, the view command discards unlikely alleles. |
Indicate PL | Indicate PL is generated by r921 or before (ordering is different). |
No genotype information | Suppress all individual genotype information. |
A/C/G/T only | Skip sites where the REF field is not A/C/G/T. |
List of sites | List of sites at which information are outputted. |
QCALL likelihood | Output the QCALL likelihood format. |
List of samples | List of samples to use. The first column in the input gives the sample names and the second gives the ploidy, which can only be 1 or 2. When the 2nd column is absent, the sample ploidy is assumed to be 2. In the output, the ordering of samples will be identical to the one in FILE. |
Min samples fraction | Skip loci where the fraction of samples covered by reads is below FLOAT. |
Per-sample genotypes | Call per-sample genotypes at variant sites. |
INDEL-to-SNP Ratio | Ratio of INDEL-to-SNP mutation rate. |
Gap open error | Phred-scaled gap open sequencing error probability. Reducing INT leads to more indel calls. |
Max P(ref|D) | A site is considered to be a variant if P(ref|D). |
Pair/trio calling | Enable pair/trio calling. For trio calling, option -s is usually needed to be applied to configure the trio members and their ordering. In the file supplied to the option -s, the first sample must be the child, the second the father and the third the mother. The valid values of STR are “pair”, “trioauto”, “trioxd” and “trioxs”, where “pair” calls differences between two input samples, and “trioxd” (“trioxs”) specifies that the input is from the X chromosome non-PAR regions and the child is a female (male). |
N group-1 samples | Number of group-1 samples. This option is used for dividing the samples into two groups for contrast SNP calling or association test. When this option is in use, the following VCF INFO will be outputted: PC2, PCHI2 and QCHI2. |
N permutations | Number of permutations for association test (effective only with -1). |
Max P(chi^2) | Only perform permutations for P(chi^2). |
Choose these parameters and click the Next button. The next page of the wizard appears:
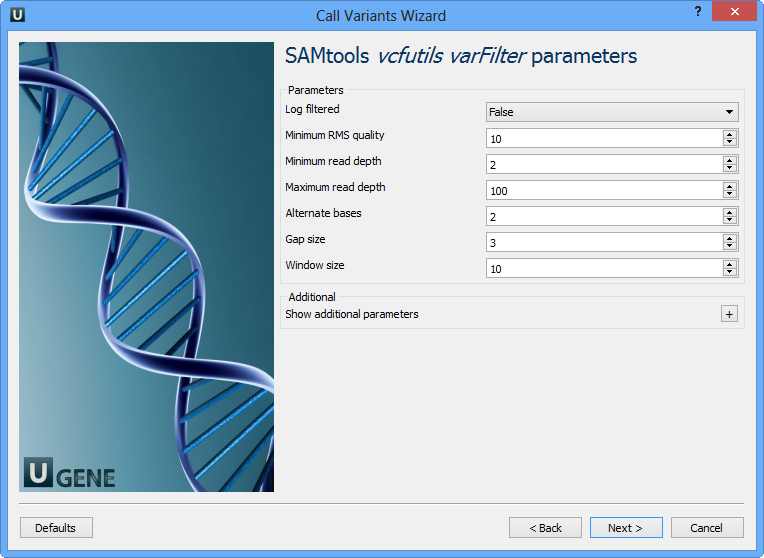
The next page allows one to configure SAMtools vcfutils parameters:
Log filtered | Print filtered variants into the log (varFilter) (-p). |
Minimum RMS quality | Minimum RMS mapping quality for SNPs (varFilter) (-Q). |
Minimum read depth | Minimum read depth (varFilter) (-d). |
Maximum read depth | Maximum read depth (varFilter) (-D). |
Alternate bases | Minimum number of alternate bases (varFilter) (-a). |
Gap size | SNP within INT bp around a gap to be filtered (varFilter) (-w). |
Window size | Window size for filtering adjacent gaps (varFilter) (-W). |
Strand bias | Minimum P-value for strand bias (given PV4) (varFilter) (-1). |
BaseQ bias | Minimum P-value for baseQ bias (varFilter) (-2). |
MapQ bias | Minimum P-value for mapQ bias (varFilter) (-3). |
End distance bias | Minimum P-value for end distance bias (varFilter) (-4). |
HWE | Minimum P-value for HWE (plus F<0) (varFilter) (-e). |
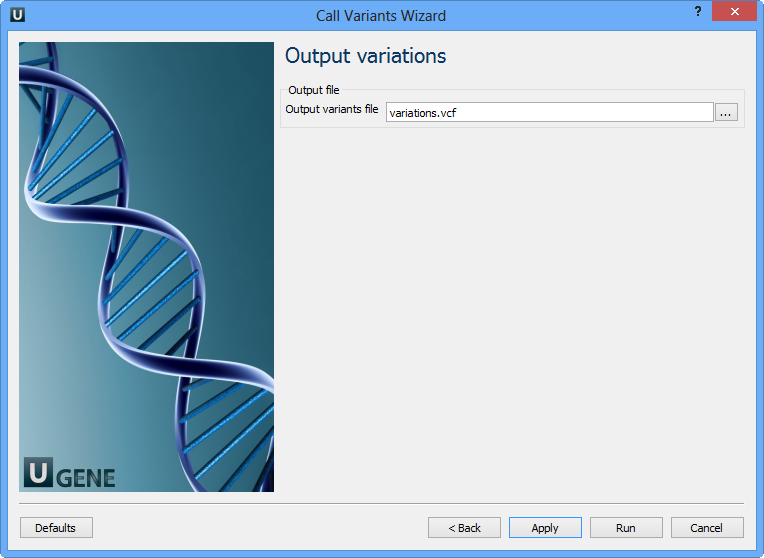
On this page you should select an output file. Set required parameters and click the Finish button.
Note that default button reverts all parameters to default settings.
Now let’s validate and run the workflow. To validate that the workflow is correct and all parameters are set properly click the Validate workflow button on the Workflow Designer toolbar:
If there are some errors, they will be shown in the Error list at the bottom of the Workflow Designer window, for example:
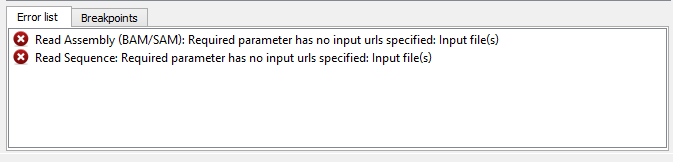
However, if you have set all the required parameters, then there shouldn’t be errors. After that you can estimate the workflow. To run estimation click the Estimate workflow button:
To run a valid workflow, click the Run workflow button on the Workflow Designer toolbar:
As soon as the variants calling task is finished, a notification and dashboard will appear.
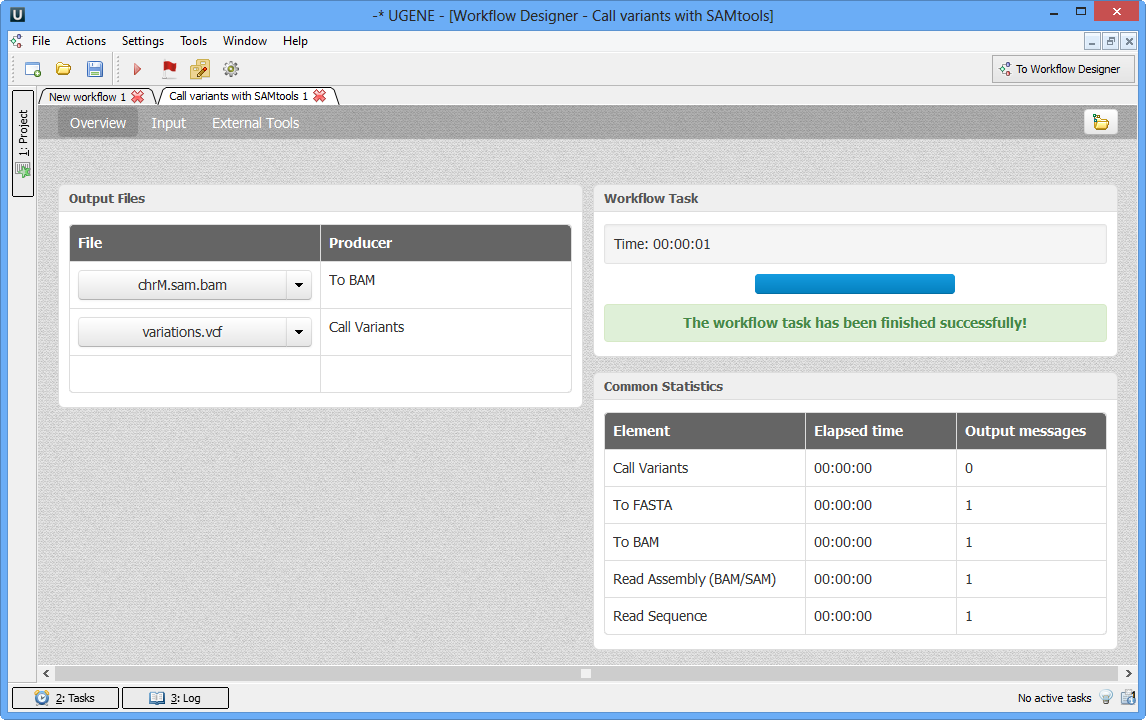
The dashboard will contain information about workflow: input and output files, all information about task. .
The work on this pipeline was supported by grant RUB1-31097-NO-12 from NIAID.